Project A2
Doctoral researcher: Henrik Müller
Principle investigator: Uwe T. Bornscheuer
Co-supervisor: U. Lendeckel, G. Daum
Functional analysis of Tafazzins for their role in Cardiolipin remodelling
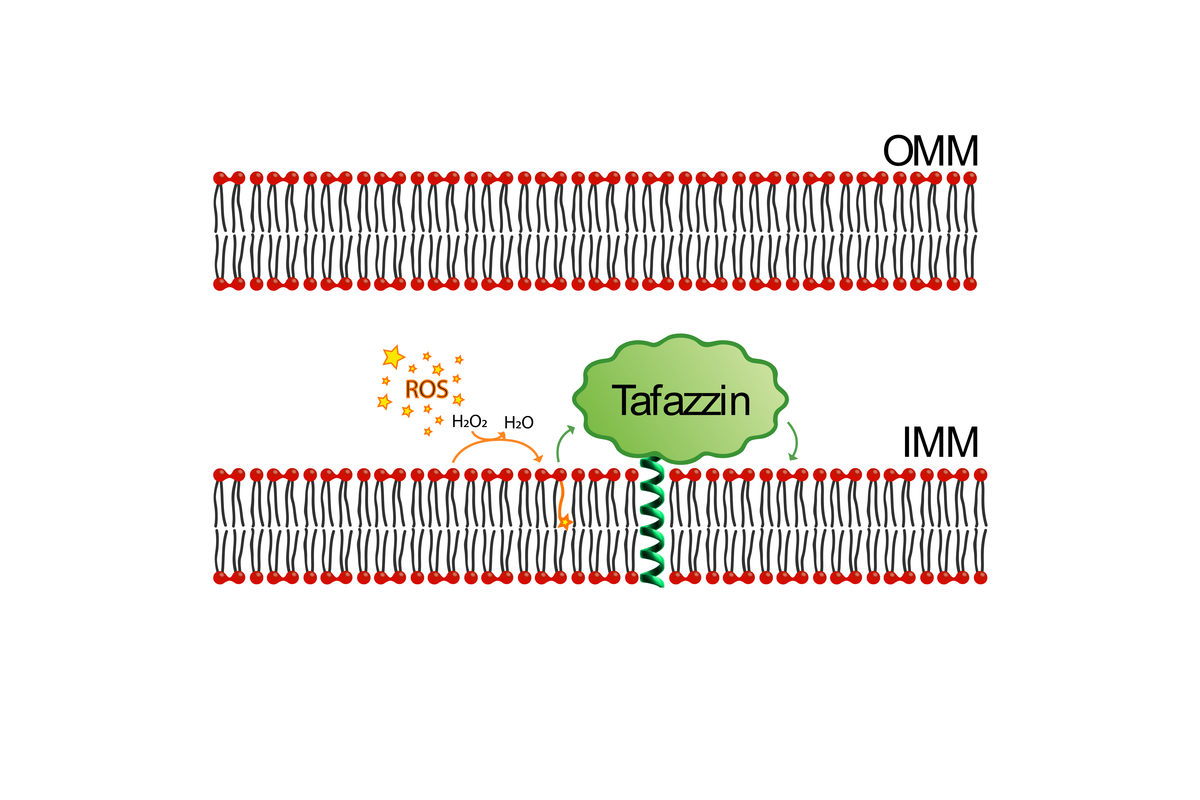
Background: Tafazzin is a phospholipid transacylase located in the inner mitochondrial membrane and is involved in the modulation of the acyl chain composition of Cardiolipin (CL), the only phospholipid specific to mitochondria.
Mutations in the human tafazzin cause the Barth syndrome[1], a disease that is connected to cardiomyopathy, skeletal muscle weakness, fatigue and abnormal growth. Although some studies have investigated tafazzins from various species, especially humans, controversy exists about the mechanism and functional implications of CL remodeling catalyzed by tafazzin(s)[2]. For human tafazzin, a preliminary study has investigated some amino acid residues in Barth syndrome causing mutations and indicated a possible role in membrane association, substrate binding and conformational stability of tafazzin[3]; another publication investigated a tafazzin from S. cerevisiae with respect to its acyl chain length preference[4]. A very recent study suggests a general role of interfacial lipids for the stabilization of membrane protein oligomers[5]. Prof. Bornscheuer has an international reputation for his research on protein engineering and protein functional analysis[6] as well as is an expert in enzymatic lipid modification[7]. A BLAST comparison of Tafazzin sequences reveals considerable differences between tafazzin proteins from mammalians, yeast and other organisms. Furthermore, within human tafazzins, point mutations are also present with limited information about their function.
Work Plan: Hence, in this project we will on one hand perform a bioinformatic analysis to identify distinct mutations present in tafazzins and to guide protein engineering of these enzymes to improve our understanding of sequence-function relationships. Furthermore, we plan to investigate the relationship between tafazzins and the composition of cardiolipin. Tafazzin variants will be studied in close collaboration with the Co-PI, Prof. Lendeckel with special emphasis given to ROS formation. This project aims to provide important insights into the metabolic role of tafazzin(s) in cardiolipin modification and its susceptibility to ROS.
Literature
[1.] (a) Claypool, S. M., et al. J Cell Biol 2011,192, 447-462; (b) Kirwin, S. M., et al. Mol Genet Metab 2014,111, 26-32.
[2.] Schlame, M.; Greenberg, M. L. Biochim Biophys Acta 2017,1862, 3-7.
[3.] Hijikata, A., et al. Meta Gene 2015,4, 92-106.
[4.] Abe, M., et al. J Biol Chem 2016,291, 15491-15502.
[5.] Gupta, K., et al. Nature 2017,541, 421-424.
[6.] (a) Bornscheuer, U. T., et al. Nature 2012,485, 185-194; (b) Kazlauskas, R. J.; Bornscheuer, U. T. Nature Chem. Biol 2009,5, 526-529; (c) Lutz, S.; Bornscheuer, U. T., Protein Engineering Handbook. Wiley-VCH: Weinheim, 2009.
[7.] Zorn, K., et al., Prog Lipid Res 2016,63, 153-164.
Contact
Uwe T. Bornscheuer
University of Greifswald
Institute of Biochemistry
Felix-Hausdorff-Str. 4
D-17487 Greifswald
Tel: +49 (0)3834 420 4367
Fax:+49 (0)3834 420 794367
uwe.bornscheueruni-greifswaldde
Website