Project B4
Doctoral researcher: Mehdi Ravandeh
Principle investigator: Heike Kahlert and Michael Lalk
Co-supervisor: K. Wende
Interaction of RNS and ROS with lipid mono- and bilayers immobilized on electrodes and protection of such layers with different radical scavengers
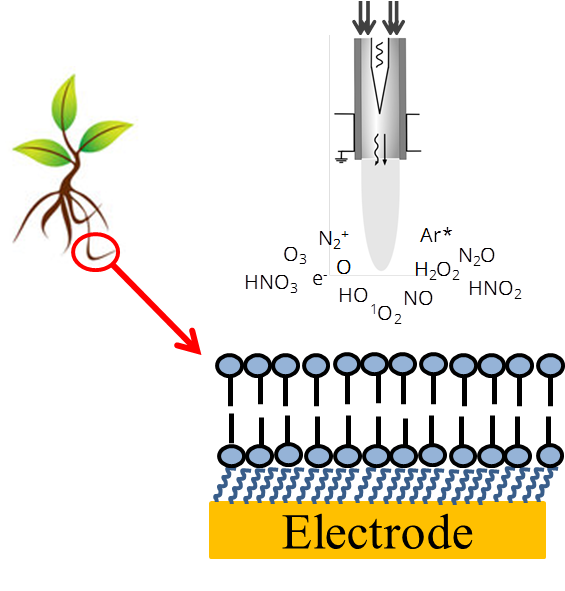
In the project we will investigate the interaction of RNS and ROS with model lipids and natural plant membranes immobilized on electrode surfaces, e. g. planar gold electrodes or mercury drop electrodes. Two questions will be addressed: i) what are the fragmentation products and ii) how specific substances well known to have radical scavenging properties can protect plant membrane components against RNS and ROS.
In cooperation with the group of Prof. Stöhr we will use phosphatidylcholine as a model lipid, plant membranes after purification (pure lipids), membranes without proteins but containing all other lipophilic substances, and at least complete natural membranes. For this purpose, plasma membranes will be obtained from plant roots and thylakoids. A lipid monolayer or bilayer on an electrode surface can serve as a simple model to investigate directly the destructive interaction of radicals with such common model of a cell membrane. To obtain lipid layers on electrode surfaces different techniques are available as self-assembly, vesicle spreading and Langmuir-Blodgett techniques [1-5].
As a result of project B4 we could establish a pre-treatment protocol for polycrystalline gold electrodes resulting in highly reproducible thiol SAMs on such gold substrates. The destruction of the layers as well as the protective effect of radical scavengers were followed by, e. g. voltammetric methods.
Continuing project B4, a combination with electrochemical measurements and state-of-the-art bioanalytical techniques like GC or HPLC in combination with mass spectrometry (GC-MS, HPLC-MS and HPLC-MS/MS) allows the analysis of membrane components after cleaning procedure, and the recording of fragmentation products of the lipids after ROS or RNS attack. To control the composition (RNS or ROS) and quantity of produced radicals, we will use low temperature plasma jet (kINPen®) [6-9]. Additionally, in combination with SECM (scanning electrochemical microscopy), structural changes of lipid layers during radical attack will be followed. The elucidation of structural modification in lipids will be complemented by NMR spectroscopic analysis using a newly installed high-sensitivity 1.7mm TCI cryo-NMR probe. Here we will employ 1H and 13C-NMR spectroscopy as well as selected 2D-NMR experiments to elucidate the structures of modified lipid metabolites.
Additionally, in cooperation with Prof. Scholz we will analyse the membrane fluidity. Furthermore, the influence of lipophilic components and proteins on the stability of lipid layers towards a ROS or RNS attack should provide a deeper mechanistic insight into interactions of radicals with lipid layers. A detailed study of the protection of different lipid metabolites depending on the nature of radical scavengers (synthetic and natural) as well as the conditions (pH, temperature etc.) under which a maximum in radical scavenging activity can be achieved is of importance in understanding the impact of radical scavengers on cell protection.
Literature
[1] M. Nullmeier, H. Koliwer-Brandl, S. Kelm, P. Zägel, K.-W. Koch, I. Brand, ChemPhysChem, 2011, 12, 1066.
[2] P. C. Gufler, D. Pum, U. B. Sleytr, B. Schuster, Biochim Biophys Acta, 2004, 1661, 154.
[3] X. Han, A. S. Achalkumar, R. J. Bushby, S. D. Evans, Chemistry, 2009,15, 6363.
[4] W. Knoll, R. Naumann, M. Friedrich, J. W. F. Robertson, M. Lösche, F. Heinrich, U. B. Sleytr, Biointerphases, 2008, 3, FA125.
[5]J. T. Marquês, R. F. M. De Almeida, A. S. Viana, Electrochim Acta, 2014, 126, 1398.
[6] S. Bekeschus, A. Schmidt, K.-D. Weltmann, T. von Woedtke, Clinical Plasma Medicine, 2016, 4, 19.
[7] K.D. Weltmann, E. Kindel, R. Brandenburg, C. Meyer, R. Bussiahn, C. Wilke, T. von Woedtke, Contrib Plasm Phys, 2009, 9, 631.
[8] K. Wende, P. Williams, J. Dalluge, W. Van Gaens, H. Aboubakr, J. Bischof, T. von Woedtke, S.M. Goyal, K.-D. Weltmann, A. Bogaerts, Biointerphases, 2015, 10, 029518.
[9] A. Schmidt, S. Dietrich, A. Steuer, K.-D. Weltmann, T. von Woedtke, K. Masur, K. Wende, J Biol Chem, 2015, 290, 6731
Contact
Heike Kahlert
University of Greifswald
Institute of Biochemistry
Felix-Hausdorff-Str. 4
D-17487 Greifswald
Tel: +49 (0)3834 420 4452
Fax:+49 (0)3834 420 4413
hkahlertuni-greifswaldde
Website
Michael Lalk
University of Greifswald
Institute of Biochemistry
Felix-Hausdorff-Str. 4
D-17487 Greifswald
Tel: +49 (0)3834 420 4867
Fax:+49 (0)3834 420 4479
lalkuni-greifswaldde
Website