Project A4
Doctoral researcher: Christina Schaal
Principle investigators: Matthias Elsner/ Sigurd Lenzen and Uwe Lendeckel
Co-supervisor: F. Scholz
Effect of free fatty acid composition of phospholipids on the hydrogen peroxide permeability of biological membranes
Reactive oxygen species (ROS) play an important role in the pathogenesis of diabetes mellitus. Type 2 diabetes (T2DM) is often accompanied by elevated plasma concentrations of long-chain free fatty acids which lead to increased levels of hydrogen peroxide (H2O2) generated in the peroxisomal β-oxidation[1;2]. Pancreatic β-cells are exceptionally sensitive to oxidative stress because of their low glutathione peroxidase and catalase expression[3]. H2O2 cannot be inactivated but can form highly toxic hydroxyl radicals in the Fenton reaction. Organelles are delineated by biological membranes which differ in their oxidation sensitivity by ROS and permeability for H2O2.
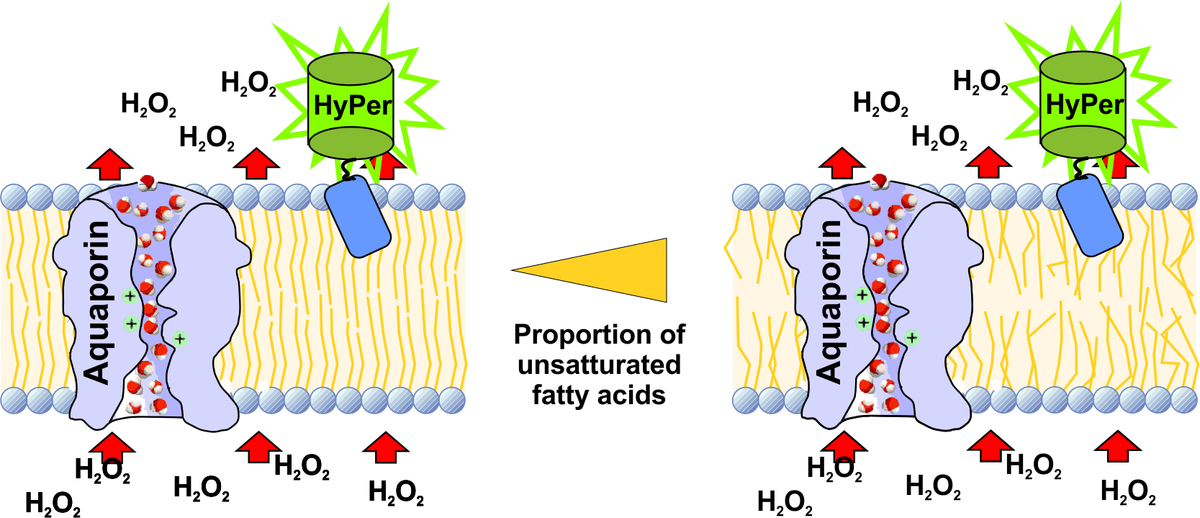
In the first three years of the RTG 1947 we developed a fluorescence microscopy-based technique with HyPer sensor proteins to analyse the H2O2 permeability of organelle membranes in living cells. Fatty acid composition of biological membranes is considered to be dependent on the dietary fatty acids and thereby affects the physicochemical properties of biological membranes [4].
For the next three years of the RTG1947 the influence of free fatty acid composition on the membrane permeability of H2O2 on the cell organelle membranes will be investigated by the use of the established HyPer-based measurement technique. HyPer expressing cells will be incubated with different fatty acids. Subsequently fluorescence changes of the membrane tagged HyPer proteins will be detected by live cell microscopy. In this context we will also characterise the expression of the different isoforms of the H2O2 transporters, the aquaporins [5] in the cellular membranes to identify how H2O2 travels from the sites of generation to the sites of toxic action within the cell.
In cooperation with U. Bornscheuer and M. Lalk the phospholipid composition of the membranes after exposure of cells against different fatty acids will be analysed by gas chromatography/mass spectrometry. In addition we intend to measure the effect of phospholipid composition on cellular elasticity (in collaboration with O. Otto) and membrane fluidity (in collaboration with F. Scholz). In cooperation with U. Lendeckel and L. Schild the mitochondrial cardiolipin composition will be analysed after free fatty acid incubation to elucidate a potential mechanism for lipotoxicity.
Experimental tools and methodological developments for use of the partners: (a) Generation of stable transgenic cell lines through lentiviral transduction. Lentiviral vector systems are highly efficient for the transduction of tissue culture cells that are difficult to transfect or mitotically inactive primary cells. The Institute of Clinical Biochemistry has many years of experience in the preparation of recombinant lentiviruses, their purification and quality control[6]. An established TetOn-system enables a regulated gene expression for potential cytotoxic gene products or to perform dose-response analyses. ShRNA based lentiviral constructs allow time saving gene kockdown experiments. (b) Fluorescence sensor proteins tagged for different subcellular sides for the detection of H2O2 (HyPer, HyPer2, HyPer Red, roGFP2-Orp1), ER-located H2O2 (TriPer) [7], redox potential (roGFP2), Ca2+(mKate2), pH (SypHer), Glucose (FLIPGlu) (c) Adenoviral or lentiviral delivery of CRISPR/Cas9. (d) Live-cell fluorescence microscopy.
Literature
[1] Elsner M, Gehrmann W, Lenzen S (2011) Peroxisome-generated hydrogen peroxide as important mediator of lipotoxicity in insulin-producing cells. Diabetes 60, 200-208.
[2] Gehrmann W, Würdemann W, Plötz T, Jörns A, Lenzen S, Elsner M (2015) Antagonism Between Saturated and Unsaturated Fatty Acids in ROS Mediated Lipotoxicity in Rat Insulin-Producing Cells. Cell Physiol Biochem 36: 852-865.
[3] Lenzen S (2008) Oxidative stress: the vulnerable beta-cell. Biochem Soc Trans 36: 343-347.
[4] Antonny B, Vanni S, Shindou H, Ferreira T (2015) From zero to six double bonds: phospholipid unsaturation and organelle function. Trends Cell Biol 25: 427-436.
[5] Bienert GP, Chaumont F (2014) Aquaporin-facilitated transmembrane diffusion of hydrogen peroxide. BBA 1840: 1596-1604.
[6] Elsner M, Terbish T, Jörns A, Naujok O, Wedekind D, Hedrich HJ, Lenzen S (2012) Reversal of diabetes through gene therapy of diabetic rats by hepatic insulin expression via lentiviral transduction. Mol Ther 20:918-26.
[7] Melo EP, Lopes C, Gallwitzer P, Lortz S, Lenzen S, Mehmeti I, Kaminski CF, Ron D, Avezov E (2017) TriPer, an optical probe tuned to the endoplasmic reticulum tracks changes in luminal H2O2. BMC Biol 15:24.
Contact
Matthias Elsner
Hannover Medical School
Institute of Clinical Biochemistry
Carl-Neuberg-Str. 1
D-30625 Hannover
Tel: +49 (0)511 532 3765
Fax:+49 (0)511 532 3584
elsner.matthias@mh-hannover.de
Website
Sigurd Lenzen
Hannover Medical School
Institute of Clinical Biochemistry
Carl-Neuberg-Str. 1
D-30625 Hannover
Tel: +49 (0)511 532 6547
Fax:+49 (0)511 532 3584
lenzen.sigurdmh-hannoverde
Website
Uwe Lendeckel
University Medicine Greifswald
Institute of Medical Biochemistry and Molecular Biology
Ferdinand-Sauerbruchstr.
D-17475 Greifswald
Tel: +49 (0)3834 86 5425
Fax:+49 (0)3834 86 5402
uwe.lendeckeluni-greifswaldde
Website