Project A1
Doctoral researcher: Clara Ortegón Salas
Principle investigator: Christopher Horst Lillig
Co-supervisors: M. Gellert, C. A. Helm, S. Bekeschus
Signal-regulated oxidation of proteins in the cytosol via a flavo-monooxygenase-disulfide relay at the cell membrane
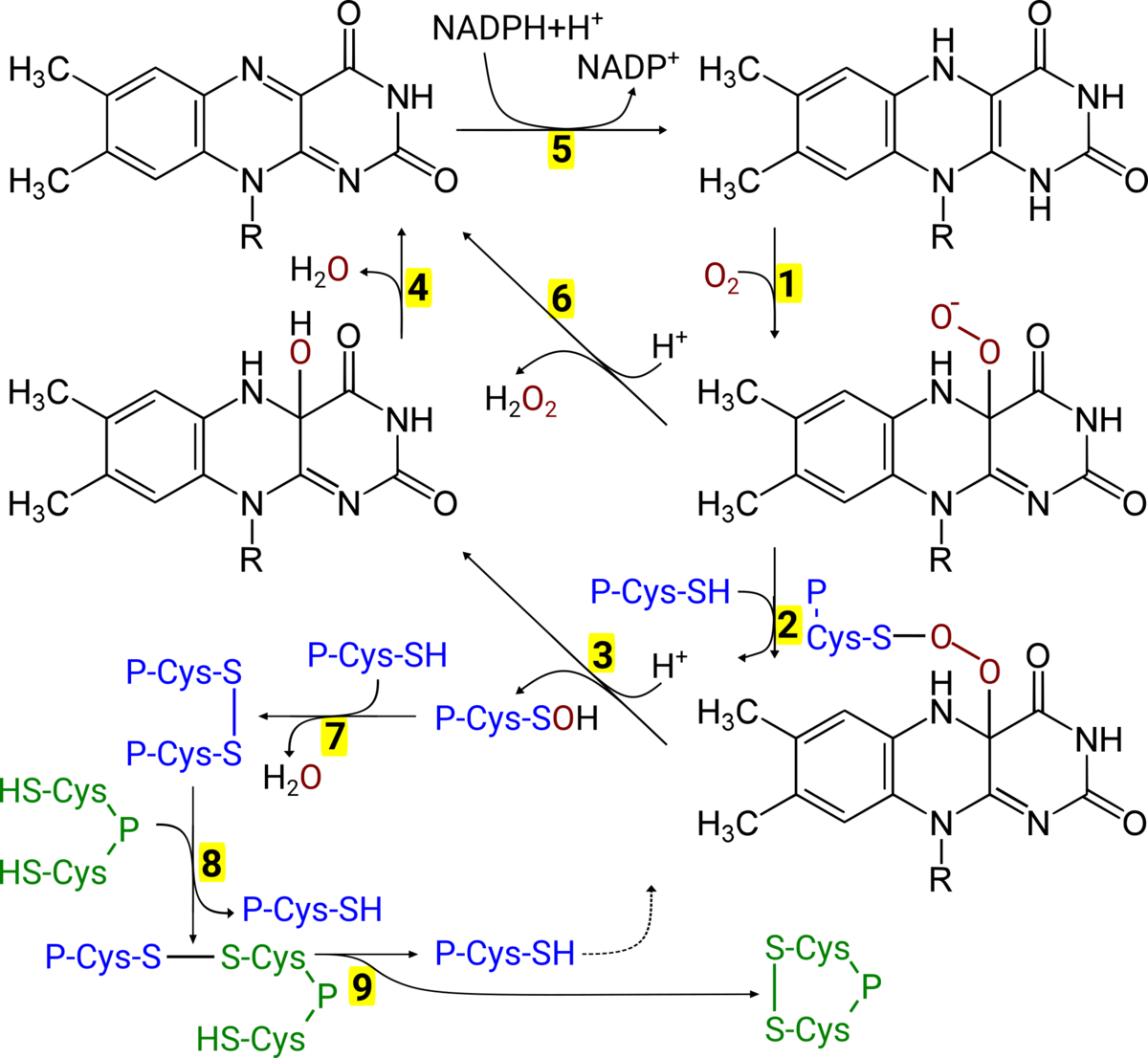
Background: The mechanisms that lead to the formation of regulatory protein disulfides (thiol switches) in the cytosol are still unresolved [1]. MICAL proteins are FAD-dependent monooxygenases that act as effector proteins in cell signalling cascades. For instance, the semaphorin (Sem) pathway, that controls neuronal development and cell migration. Sem3A binds to its receptor on the cell surface, that consists of neuropilin 1 and plexin A. This signal is transferred through the membrane and leads to the activation of MICALs. Activated MICAL can produce H2O2, (see Fig. 1 reactions 1→6→5) [2], however a direct oxidation of methionyl residues of the protein beta-actin has also been described [3].
Preliminary results: In a previous project (DFG Li 984/3-1), we have demonstrated that MICAL may also directly oxidize proteins of the peroxiredoxin family (Prx, see Fig. 1, reaction 3).
Hypothesis: Here, we propose the following model for the Sem3A-induced oxidation of protein thiols at the cell membrane (Fig. 1). MICAL with reduced FADH2 reacts with O2 to form a peroxy-flavin (reaction 1). This peroxy-group is attacked by the catalytic thiol in the active site of the Prx (2) to form a covalently linked intermediate. This complex dissociates to form H2O and a sulfenic acid in the active site of the Prx (reaction 3). By attack of a resolving cysteine of a second Prx molecule, this is reduced to H2O, leaving a disulfide between the Prx molecules (7). The flavin is regenerated in two steps (4→5) by NADPH. The disulfide on the Prx is subsequently transferred to the target protein in a thiol-disulfide exchange reaction (8→9).
Plan of work: The first work package is a detailed analysis of the reaction proposed in Fig. 1 in vitro. We will use recombinant proteins and apply enzyme kinetic measurements and spectroscopic analysis (UV-vis, fluorescence, aerobic/anaerobic, and stopped flow analysis) as well as electrophoretic separation of the proteins to identify each individual intermediate species. The second work package aims at confirming this redox-signalling relay in vivo, i.e. in cell cultures. Next to confirming the sequence of events, we will use the analysis of cell migration by live cell analysis as biological read out. This program requires the support of other RTG groups and collaboration partners, e.g. Bekeschus, Bornscheuer, and Helm.
Literature
[1] M. Deponte, C.H. Lillig, Enzymatic control of cysteinyl thiol switches in proteins, Biol. Chem. 369 (2015) 401-13.
[2] E.F. Schmidt, S.-O. Shim, S.M. Strittmatter, Release of MICAL autoinhibition by semaphorin-plexin signaling promotes interaction with collapsin response mediator protein, J. Neurosci. Off. J. Soc. Neurosci. 28 (2008) 2287–97.
[3] M. Gellert, E.-M. Hanschmann, K. Lepka, C. Berndt, C.H. Lillig, Redox regulation of cytoskeletal dynamics during differentiation and de-differentiation, Biochim. Biophys. Acta BBA - Gen. Subj. 58 (2014) 1575-87.
Contact
PD Dr. Christopher H. Lillig (Vice Speaker)
University Medicine Greifswald
Institute for Medical Biochemistry and Molecular Biology
Sauerbruchstr.
DE-17475 Greifswald, Germany
Tel: +49 (0)3834 86 5407
Fax:+49 (0)3834 86 5402
lilligc(at)uni-greifswald(dot)de
Website